Avigan, approved for manufacture and sale in Japan as an influenza antiviral drug, selectively inhibits RNA polymerase necessary for influenza virus replication. Due to this mechanism of action, it has been expected that Avigan may have an antiviral effect on the novel coronavirus, as they are RNA viruses of the same type as influenza viruses. FUJIFILM Toyama Chemical conducted phase III clinical trial in Japan in March of this year for COVID-19 patients with non-severe pneumonia. The company confirmed, with a statistically significant difference, that the administration of Avigan demonstrates shorter time to resolution, with no new safety concerns identified.
Based on the results of this trial, FUJIFILM Toyama Chemical filed an Application for Partial Changes to manufacturing and marketing approval matters of Avigan. The filing seeks to add, indication as well as dosage and administration concerning COVID-19 to the current manufacturing and marketing approval items of Avigan.
To meet the requests of the Japanese government to increase stockpiles of Avigan, and by other countries to supply the drug, the Fujifilm Group has been working to increase production of the drug in collaboration with strategic partners both inside and outside Japan. The Fujifilm Group will work to deliver the treatment drug to COVID-19 patients as soon as possible, and contribute to ending the spread of COVID-19.
<About Avigan® Tablets>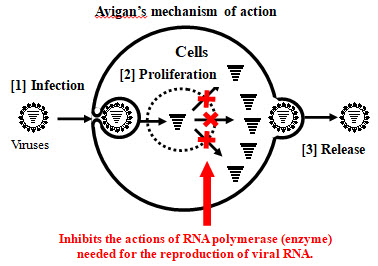
An anti-influenza drug developed by FUJIFILM Toyama Chemical that obtained domestic manufacturing and marketing approval in March 2014 with the treatment of new or reemerging influenza as the indication. The Japanese government has already stockpiled Avigan in preparation for the outbreak of the novel influenza.
For inquiries about this media release, please contact:
<Media>
FUJIFILM Holdings Corporation
Corporate Communications Division, Public Relations Group
Phone: +81-3-6271-2000
###
